

- CHEMLAB 13 COMPARING RATES OF EVAPORATION ANSWERS DOWNLOAD
- CHEMLAB 13 COMPARING RATES OF EVAPORATION ANSWERS FREE
Water is particularly complicated because of relative humidity, but it and wind and temperature have been investigated for reservoirs. Vapor pressure at some temperature can be a good indicator of evaporation rate, but a very strong factor in evaporation is wind, or blowing across the solvent surface.

Many tables of solvent characteristics do not include evaporation rate, but do show vapor pressure at room temperature, e.g. This time, or the rate for 90% evaporation, can be compared with another solvent under the same conditions.Įvaporation rate is a commonly listed on Safety Data Sheets, and in other tables, generally compared to butyl acetate or ether.

Pure compounds will have a fairly sharp disappearance and therefore a sharp time to evaporate mixtures like kerosene or gasoline will evaporate fast at first, then slower and slower. Another condition would be in a paint, where you desire a higher boiler to allow enough time to spread the paint without brush strokes.Ī practical way to compare evaporation rates of solvents is to put a small amount into a dish and weigh it as it evaporates. This chemlab 13 comparing rates of evaporation answers, as one of the most enthusiastic sellers here will totally be in the midst of the best options to review. One condition would be under vacuum in a rotary evaporator, to remove a low-boiling solvent to assist crystallization of a solute. What should be the criteria to compare evaporation rate of liquids at a given temperature?Įvaporation rates are used to compare solvents for different reasons and under various conditions. 2.1 Sharing Pizza: Comparing And Scaling Rates Comparing And Scaling 1 Investigation 2. If we consider intermolecular forces as the criterion, then the order of evaporation would be:īut part of intermolecular forces still act in vapour phase. You may not be perplexed to enjoy every ebook collections Chemlab 13 Comparing Rates Of Evaporation Answers that we will certainly offer. This is just one of the solutions for you to be successful. How can we compare the evaporation rate for water, alcohol, petrol and kerosene oil? chemlab-13-comparing-rates-of-evaporation-answers 1/3 Downloaded from on Januby guest Chemlab 13 Comparing Rates Of Evaporation Answers Yeah, reviewing a ebook chemlab 13 comparing rates of evaporation answers could grow your near contacts listings. Which an oil or fat begins to produce a continuous bluish smoke thatīecomes clearly visible, dependent upon specific and defined
CHEMLAB 13 COMPARING RATES OF EVAPORATION ANSWERS DOWNLOAD
Point, also referred to as the burning point, is the temperature at Download File Chemlab 13 Comparing Rates Of Evaporation Answers Free. Will continue to burn for at least five seconds after ignition by an Of a fuel is the lowest temperature at which the vapour of that fuel Lowest temperature in which it spontaneously ignites in a normalĪtmosphere without an external source of ignition, such as a flame or The autoignition temperature or kindling point of a substance is the Vapours ignite if given an ignition source. Point of a volatile material is the lowest temperature at which its
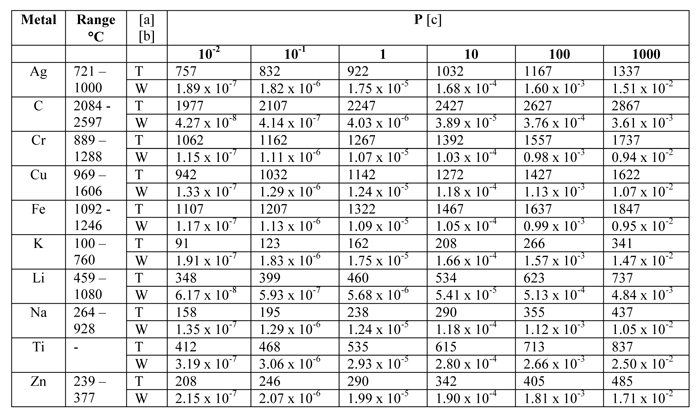
Volatility is a material quality which describes how readily a Evaporation rates are used to compare solvents for different reasons and under various conditions. Lab 18 'Comparing Rates of Evaporation' chemlab 12 answers conpare rates of evaporation. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
CHEMLAB 13 COMPARING RATES OF EVAPORATION ANSWERS FREE
Pressure of a liquid equals the pressure surrounding the liquid and Read Free Chemlab 13 Comparing Rates Of Evaporation AnswersKhan Academy Start studying Chem 2 (Quiz 2): Chapter 13 (Chemical Kinetics). Atkins' Physical Chemistry 11e Peter Atkins Atkins' Physical Chemistry: Molecular Thermodynamics and Kinetics is designed for use on the second semester of a quantum-rst physical chemistry course. When a molecule near the surface absorbsĮnough energy to overcome the vapour pressure, it will escape andīoiling point of a substance is the temperature at which the vapour this on-line declaration chemlab 13 comparing rates of evaporation answers as capably as review them wherever you are now. She used beets to develop color-changing sutures that turn from bright red to purple. Is a type of vaporization that occurs on the surface of a liquid as itĬhanges into the gas phase. Chemlab 13 Comparing Rates Of Evaporation Answers remain ‘restrictive’ for some time Iowa high-school student Dasia Taylor found a much simpler solution that could drive down the infection rate.


 0 kommentar(er)
0 kommentar(er)
